Recommending
This working group supports efforts to make evidence-based recommendations – in the context of both technology assessments and guidelines – in ways that are more coordinated and efficient and that balance quality and timeliness.
Terms of reference (with 1-3 being about identifying, 4-6 about collaborating, and 7 about undertaking)
- Provide an initial overview of emerging and existing repositories and initiatives for COVID-19 guidance (completed and underway), through mapping and surveying initiatives and organizations
- Identify and support the most useful repository for trustworthy COVID-19 guidance (the ‘global guidance repository’) that can be re-used, shared and adapted globally and is optimally linked to other repositories of evidence sources (e.g., systematic reviews, evidence tables, economic models) from trustworthy partners such as PAHO, WHO, G-I-N, and others)
- Identify and share standards, methods, processes and digital platforms for developing, disseminating, adapting and implementing trustworthy, actionable and living guidance (linked to evidence)
- Collaborate with key organizations within the technology assessment and guideline fields to share their COVID-19 guidance and evidence tables to initially feed into COVID-END repository and ultimately the global guidance repository
- Contribute to maintaining the guide to COVID-19 evidence sources and encourage its use to avoid unnecessary duplication, while coordinating with the global guidance repository
- Connect other COVID-END working groups to guidance activities to reduce duplication and facilitate work among groups (with emphasis in digitizing, synthesizing, and packaging groups)
- Conduct or support efforts to conduct quality assessments of the available guidelines (to add to the global guidance repository)
Participants
- Ivan Florez, AGREE Collaboration, University of Antioquia, Colombia (co-chair)
- Per-Olav Vandvik, MAGIC Evidence Ecosystem Foundation, Norway (co-chair)
- Alric Ruether, Institute for Quality and Efficiency in Health Care (IQWiG), Germany
- Amir Qaseem, American College of Physicians, U.S.A.
- Elie Akl, Systematic Review Centre for Health Policy and Systems, AUB, Lebanon
- Ignacio Neumann, Epistemonikos, Chile
- Jerry Osheroff, ACTS, U.S.A.
- Michael McCaul, Centre for Evidence-based Health Care, Stellenbosch University, South Africa
- Mireille Goetghebeur, Institut national d'excellence en santé et en services sociaux (INESSSS), Canada
- Lucy Henry, Health Technology Assessment International (HTAi), Canada
- Ludovic Reveiz, Pan American Health Organization
- Sandra Zelman Lewis, AHRQ evidence-based Care Transformation Support (ACTS) Initiative, USA
- Susan Norris, World Health Organization
- Tamara Kredo, South African Medical Research Council, South Africa
- Xuan Elody, Evidence-Based Medicine Center, China
- Zack Munn, G-I-N, Australia
- Secretariat: Safa Al-Khateeb, McMaster Health Forum and David Tovey, COVID-END Secretariat
Meeting documents
| Meeting date | Documents |
| September 17, 2021 | |
| July 16, 2021 | |
| June 4, 2021 | |
| March 26, 2021 | |
| February 26, 2021 | |
| February 12, 2021 | |
| January 29, 2021 | |
| January 15, 2021 | |
| December 4, 2020 | |
| November 6, 2020 | |
| October 23, 2020 | |
| October 9, 2020 | |
| October 2, 2020 | |
| September 25, 2020 | |
| September 16, 2020 | |
| September 11, 2020 | |
| August 28, 2020 | |
| August 21, 2020 | |
| August 14, 2020 | |
| July 24, 2020 | |
| July 17, 2020 | |
| July 10, 2020 | |
| June 26, 2020 | |
| June 19, 2020 | |
| June 12, 2020 | |
| June 5, 2020 | |
| May 29, 2020 | |
| May 22, 2020 | |
| May 15, 2020 | |
| May 8, 2020 | |
| April 29, 2020 | |
| April 28, 2020 |
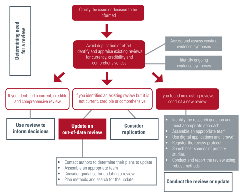
Use the interactive flow diagram to find resources for researchers considering and conducting COVID-19 evidence syntheses.
View the interactive flow diagram