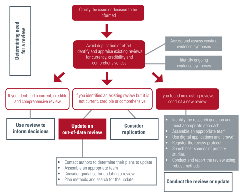
Conduct and report the review using robust methods
Methods for conducting rapid reviews
In some cases, the decision around the type of study is determined by circumstances or the expectations of the funders or sponsor. This has led to a rapid rise of 'rapid reviews.'
|
Cochrane
McMaster Health Forum
National Collaborating Centre for Methods and Tools
Alliance for Health Policy and Systems research |
Methods for conducting scoping reviews
|
Joanna Briggs Institute |
Methods for conducting systematic reviews
|
Cochrane’s mission is to promote evidence-informed health decision-making by producing high-quality, relevant, accessible systematic reviews and other synthesized research evidence
The Campbell Collaboration is an international social science research network that produces high quality, open and policy relevant evidence syntheses, plain language summaries, and policy briefs
Joanna Briggs Institute is an international research organization that develops and delivers evidence based information, including systematic reviews, software, education and training designed to improve healthcare practice and outcomes |
Methods for conducting living systematic reviews
In the context of COVID-19, there are many questions where the evidence base is expanding rapidly. Living systematic reviews aim to ensure that completed reviews do not rapidly become out of date. Not all subjects or research question are appropriate for a living systematic review, and the speed of updating will inevitably vary to suit the context and research question. Living systematic reviews generally represent questions that are judged to be likely to have an important impact on decisions, where the evidence base is unstable and moving quickly, and where conclusions are vulnerable to changing. In order to designate a systematic review as ‘living’ the following criteria are needed: active monitoring of the evidence, real-time incorporation of new data, and the ability to communicate the review status and make visible the new data that have been added (https://community.cochrane.org/review-production/production-resources/living-systematic-reviews#what). Living systematic reviews characteristically make use of digital technology or crowd-sourcing to support the process.
Guidance for reporting of the review
PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), and extensions apply to the following:
- PRISMA-P: Reporting of protocols
- PRISMA-A: Reporting of abstracts
- PRISMA-DTA: Reporting of reviews of diagnostic test accuracy
- PRISMA-ScR: Reporting of scoping reviews
- PRISMA-CI: Reporting of complex interventions
- PRISMA-E: Reporting of equity issues
- PRISMA-Harms: Reporting of harms
- PRISMA-IPD: Reporting of systematic reviews and meta-analyses of individual participant data
- PRISMA-NMA: Reporting of network meta-analyses
Once a review has been published, consider notifying the groups listed here of the completed review and consider uploading the review data into a data registry such as Figshare or SRDR to facilitate reuse of data.
Download a PDF of 'Resources for researchers considering and conducting COVID-19 evidence syntheses'
View the interactive flow diagram