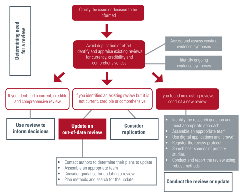
Identify existing evidence syntheses
Identifying the evidence syntheses that already exist is an essential first step to avoid duplication of effort and research waste. COVID-END has identified many of the most important searchable databases that already include published systematic reviews. If you are working directly with policymakers and other decision-makers, it may be helpful to point to work that has already been reviewed that they can consider for their own context.
|
Databases with access to variety of reviews across specific organizations
- COVID-19+ by McMaster PLUS - Critically appraised systematic reviews and single studies organized by quality level and document type
- Evidence Aid - Summaries of systematic reviews that may be relevant to COVID-19 in eight broad areas (infection prevention and control; clinical characterization and management; therapeutics and vaccines; public-health interventions; health systems and services; epidemiology; ethical considerations; and social science in response).
- L*VE by Epistemonikos - Systematic reviews of effects and the primary studies, including trials, that were included in the reviews
- LitCovid from PubMed - Systematic reviews and single studies organized by mechanism, transmission, treatment, case report, and epidemic forecasting
- TRIP database - Systematic reviews and single studies organized by document type
- U.S. Veterans’ Affairs (VA) Evidence Synthesis Program - Inventory of living (and regular) systematic reviews and ‘rapid reviews’ (completed and in progress), with a flag for living reviews and for reviews meeting minimum quality standards
Full systematic reviews (and derivative products) from specific organizations
- AHRQ EPC Program - Systematic reviews and living systematic reviews
- Campbell Collaboration - Blog profiling Campbell reviews that are relevant to COVID-19; Campbell reviews cover areas such as social welfare, crime, international development and education amongst others
- Cochrane - Special collections of Cochrane systematic reviews relevant to COVID-19 and prioritized Cochrane systematic review updates (same page as above but lower down the page; the rapid reviews are listed in the relevant section below) - Cochrane reviews addressing questions that are relevant to health care and its delivery.
- JBI - JBI Evidence Summaries provide a summary of the best available evidence related to a clinical topic, including best practice recommendations to help clinicians mobilize evidence into practice, and JBI Recommended Practices provide standardized, detailed descriptions of best practice care procedures
Other useful sources of synthesized data
- DistillerSR - Curated, tagged and downloadable references to single studies
- Health Systems Evidence and Social Systems Evidence - Systematic reviews and economic evaluations about health- and social-system arrangements presented with their focus on or relevance to COVID-19, quality rating, recency of search, and countries where the research was conducted
- Literature Review - Manually identified systematic reviews and single studies organized by topic and medical specialty
- McMaster Optimal Aging Portal - Citizen-targeted summaries of systematic reviews that may be relevant to staying active and engaged while practicing physical distancing
- SRDR - Underlying data from individual studies included in a systematic review
|
Access Grading of Recommendations, Assessment, Development and Evaluations (GRADE) evidence profiles
Some guidelines bodies and decision-makers favour the efficient production of GRADE evidence profiles rather than systematic review reports. GRADE evidence profiles describe the results of a given review, focussing on the main outcomes of interest for a given comparison of interventions. They report the direction and magnitude of any effect and the degree of certainty that an effect estimate reflects the true effect, using pre-determined criteria.
- COVID-NMA includes full evidence profiles for all comparisons of pharmaceutical, non-pharmaceutical treatments, preventive and rehabilitation interventions.
-
L*OVE Epistemonikos includes interactive summary-of-findings tables for its new 'living evidence syntheses'
|
Assess existing evidence syntheses
There are a number of resources that can be used to evaluate the credibility of published reviews or evidence syntheses. Rohwer et al provides a short guide on finding, reading and interpreting systematic reviews, as well as applying the results.
Rohwer A et al. Reading systematic reviews to answer clinical questions. Clinical Epidemiology and Global Health 2014; 2:3 9-46
- AMSTAR 2 tool: A critical appraisal tool for systematic reviews that include randomised and/or non randomised studies of healthcare interventions
- ROBIS tool
A tool to assess risk of bias
- National Collaborating Centre for Methods and Tools (NCCMT) Quality Assessment Tool: This includes a one page assessment checklist followed by a ‘quality assessment tool dictionary’ that provides guidance on how to answer each question.
|